Promising new technologies can suddenly make the future look a lot less gloomy. Today part 1: turning salt water into sweet water in a way that is not only affordable to countries like the US and Saudi-Arabia.
Today, 2.1 billion people lack safe drinking water at home, a figure that is expected to increase. (UN) Our water use is growing twice as fast as our population growth. More and more regions are reaching the limit of being able to deliver sustainable water services. If nothing changes, the projection is that by 2050 at least 1 in 4 people will be affected by recurring water shortages. (UN)
For this reason, 193 countries committed in 2015 to Sustainable Development Goal #6: access to safe water and sanitation for all by 2030. Not an easy goal to pursue. The world is facing severe water challenges: more droughts, melting ice caps, pollution, lack of water infrastructure, growing bio-energy demands, growing meat demands, and endangered ecosystems.
But here is an intriguing fact: most countries have a coast line and therefore direct access to plenty of salt water. Shouldn’t we consider desalination (turning salt water into sweet water) one of the most obvious solutions to the scarcity issue? Yes, we should. And as a matter of fact, there are already over 18,000 water desalination plants operating in 150 countries, producing water for 300 million people. (PNAS, 2017)
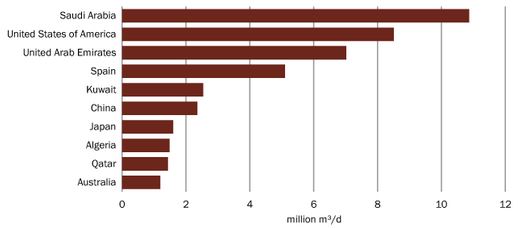
Desalination plants, however, cost a lot of money (up to 1 billion USD) and require a lot of energy: producing a 1000 liter of drinking water takes as much energy as the average Belgian consumes each day. Some countries, especially those with large oil reserves, can cope with these demands (50% of Saudi-Arabia’s drinking water comes from desalination). For other countries it is soon too much. Most of the desalination plants are therefore only in a few countries (see below).
To make desalination more affordable, researchers have been looking for ways to reduce the energy costs and make the process less dependent on expensive and immobile desalination plants. This has led to some promising innovations. In this blog I would like to highlight two of them:
- A team of Rice University (Houston, US) managed to reduce energy costs by using low-cost, commercially available nanoparticles and sunlight in the desalination process. At the same time, they turned the process into a compact water solution for families and communities also at remote locations. (PNAS, 2017) In other words: no 1 billion USD plant needed. Professor Qilin Li : “We are creating off-grid systems to provide water anywhere it’s needed.” The video below explains.
- Marjolein Vanoppen of Ghent University (Belgium) found a way to both generate energy and lower the amount of energy needed to produce drinking water. How: by benefiting from the fact that when salt and fresh water come together, the salt moves towards the fresh water, a movement that generates energy. In Vanoppen’s solution, energy is first generated by allowing salt to move from salt water to waste water (not suitable for drinking water production). The generated energy is then used to further desalinate the salt water until it is drinkable. In the video below, Vanoppen explains (from 49:03).
Promising innovations like these require further research and investment funds for scalable applications. A perfect opportunity for governments and entrepreneurs to demonstrate the visionary leadership this world so profoundly needs.
